Quercetin
Quercetin, derived from the Latin word “Quercetum” is a chemical compound found in many plants but cannot be synthesized by humans. When isolated from its plant source, the compound appears yellow in color and is insoluble in highly polar solvents like water. However, it can dissolve in ethanol and the most popular bioflavonoids that are used to cure a variety of illnesses. The highest concentration of this flavonoid is found in onion, (28.4–48.6 mg/100 g) The pharmacological importance of quercetin are
Effects of dietary quercetin on cardiovascular disease
Onion and shallot contain the major components of quercetin as 3,4′-O-β-diglucoside and quercetin 4′-O-β-glucoside. Due to the presence of two glucosidal groups around the 4′-position, are exclusively found similar in onion and shallot. The aforementioned compounds increase the role of enzymes like oxidative/antioxidative for the guideline of induced oxidative stress. In experiments with rats,0.2% of onion peel extract with high content of fat diet for 2 months on a daily basis. The results show that lowered levels of LDL and increased HDL levels. Consequently, the preferred level of the diet with onion as an efficient ingredient is used to control vascular dysfunction and atherosclerosis.
Anti-inflammatory Activity
When our bodies attempt to cure themselves, sections of them become inflamed as a biological reaction to damaging or irritating stimuli brought on by bacteria, fungi, or viruses. One of quercetin’s most notable characteristics is its capacity to control inflammation by blocking inflammatory enzymes like cyclooxygenase and lipoxygenase. Studies revealed that in the Wister rat model, administration of extract made from water on red onion (150 and 300 mg/kg) decreases the eosinophil and lymphocyte counts in the blood. However, the ethanol extract with 50, 250, and 500 µg/mL exhibits a protective effect on neuroinflammation.
Neurodegenerative disorders
When coupled with ascorbic acid, quercetin is proven to suppress neuronal damage and lower the percentage of oxidative damage to skin neurovascular cells and human lymphocytes. Individually, quercetin does not show a significant benefit against neurogenerative illnesses. Thus, quercetin combats oxidative stress and shields brain cells from Alzheimer’s and other neurological diseases. People who eat raw onions without breaking down the polyphenolics are therefore less likely to suffer from strokes linked to neuroinflammatory illnesses of the central nervous system.
Cancer
The National Cancer Institute, United States in 2019 claimed that increased exposure to Hexavalent Chromium Compounds result in high risks of lung cancer and paranasal sinuses and nasal cavity. In human colon cancer Caco-2 cells, are a chemical carcinogen to promotes cell transformation, including loss of cell visibility, production of ROS, and elevation of MicroRNA-21. In vitro and in vivo studies revealed that quercetin is beneficial against prostate cancer.
Combination treatment of curcumin(a major constituent of turmeric) and quercetin has been studied for rats by feeding curcumin 480 mg orally 3 times a day and along with quercetin 20 mg for an average of 6 months. As a result, curcumin and quercetin reduce the ileal and rectal adenomas with minimal adverse effects.
Ulcer and gastritis
Quercetin functions as a gastroprotective agent by preventing the production of gastric acid and lipid peroxidation in gastric cells. Quercetin has been shown to have positive anti-ulcer activity based on its antiulcer impact in rats exposed to ethanol-induced gastric mucosal injury model at doses of 50 and 100 mg/kg. Because it scavenges free radicals and increases the formation of stomach mucus, quercetin has anti-ulcer effects.
Structural Analysis of Quercetin
The chemical structure of a compound provides an idea about its pharmacological activity. With the knowledge of its functional groups, conformation, and electron donating capability is an important parameter to elucidate the structure-activity relationship. The presence of five hydroxy groups pentahydroxyflavone at the 3-, 3′-, 4′-, 5-, and 7-positions (figure) is responsible for the possibility of H-atom transfer. In lipid media, the proton-coupled electron transfer mechanism transfer of H-atom from quercetin’s catholic OH groups is predominant.
In gas and solvent phases, theoretical simulations are carried out using density functional theory for quercetin and its glucosides. It is concluded that the thermodynamically preferred mechanism of the antioxidative progress can be altered by the environment. Hydrogen atom transfer is the thermodynamically dominant process in the gas phase. In ethanol and water environments, sequential proton loss followed by electron transfer is more favored.
In the gas phase and solvent phase, shows that -OH groups in B-ring and C-ring contribute mainly to the antioxidative activities of quercetin and glucosides compared with A-ring.
These studies deepen and elevate our understanding of the antioxidative capacity of quercetin and its glucosides in studied phases. It may also shed light on the studies of flavonoids and glucosides in the studied environments and other solvents in the future.
Discussion
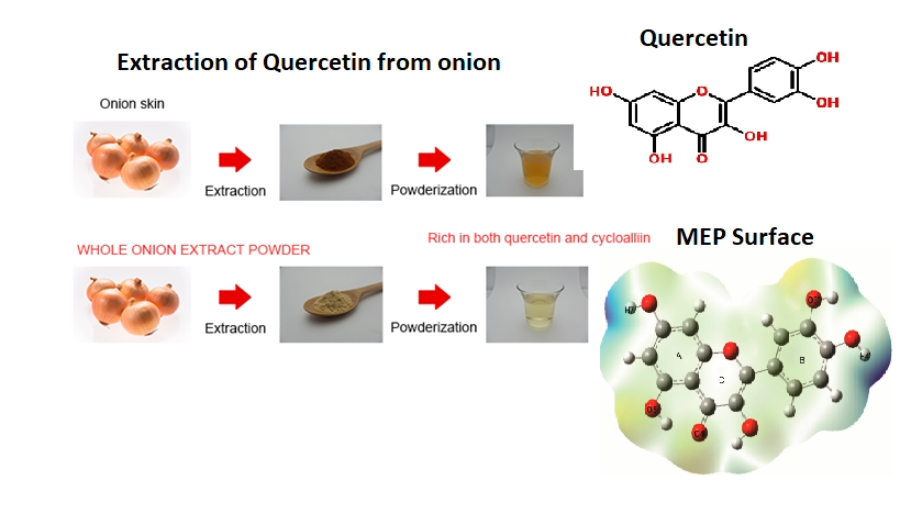
Organic compounds from plants are known to exhibit several pharmacological activities. Onion is a commonly used ingredient in South Indian foods. Yet the complete pharmacological impact has been analyzed only recently based on its structure-activity. A compound prominently found in onion is quercetin – a flavonoid. It can be extracted directly from a full onion or from its skin using polar solvents. The quercetin-rich extract/pure quercetin is found to be effective against cardiovascular diseases, neurodegenerative disorders, oxidative stress, and gastric problems. The reason is the five hydroxyl groups, particularly the –OH groups from the B-ring of the compound act as good radical scavengers. Quercetin and its derivatives either in its pure form or combined form exhibit excellent medicinal activities
Source