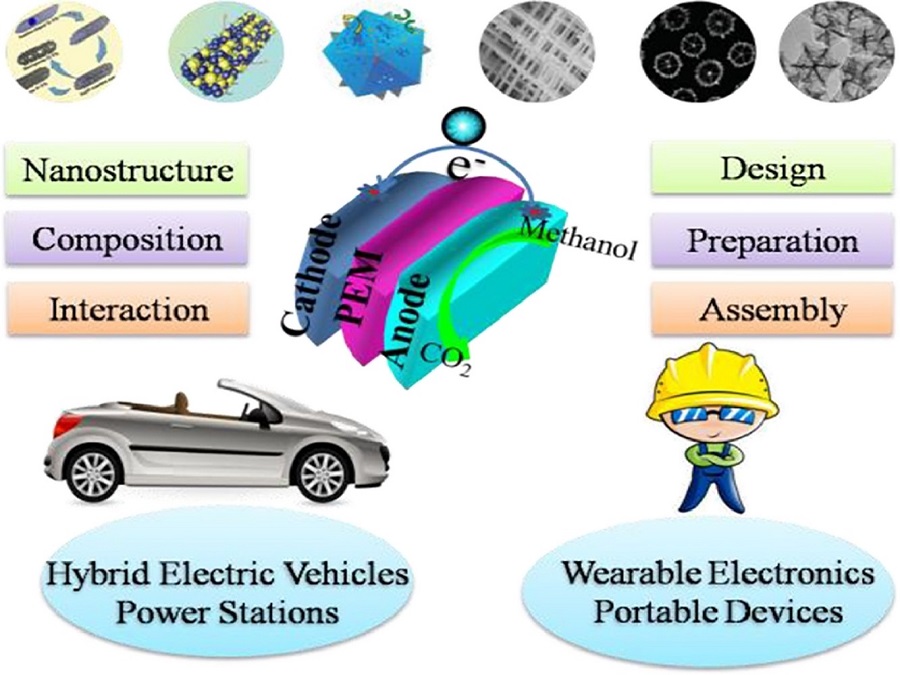
Nano architected Catalysts: Revolutionizing DMFC Efficiency and Performance
The scarcity of energy leads to technological improvements in the conversion and storage for future implementations. Fuel cells were initially documented by Grove in the early 19th century, over 180 years ago; however, it wasn’t until the early 1960s that they garnered significant attention for power generation. Operating akin to a basic galvanic cell, a fuel cell involves the continuous supply of chemicals (fuel and oxidant) to electrodes, converting the chemical energy of the fuel into electricity with minimal greenhouse gas emissions. Unlike batteries or traditional supercapacitors, fuel cells operate as open systems, with masses undergoing redox reactions supplied externally to the cell. Therefore, fuel cells are given vital importance as high-energy systems suitable for a range of applications, both stationary and mobile.
According to their operating temperature, efficiency, applications, and costs, fuel cells are of different categories. Primarily based on the choice of fuel and electrolyte, they are classified into six major groups: (i) Proton-exchange membrane fuel cell (PEMFC): (a) Direct formic acid fuel cell (DFAFC); (b) Direct ethanol fuel cell (DEFC), (ii) Alkaline fuel cell (AFC): (a) proton ceramic fuel cell (PCFC); (b) Direct borohydride fuel cell (DBFC), (iii) phosphoric acid fuel cell (PAFC), (iv) Molten carbonate fuel cell (MCFC), (v) Solid oxide fuel cell (SOFC), and (vi) Direct methanol fuel cell (DMFC).
Direct methanol fuel cell (DMFC)
Methanol is the simplest alcohol and its electrochemistry is also the easiest. The advantages such as low cost related to other fuels, comfortability of handling through transportation, ease of store, and especially much higher theoretical energy density (4.8 kW h L-1) compared to gasoline are endorsed methanol as a better fuel for DMFC applications. It operates within the temperature range of 50-120 °C.
In this regard, China started a methanol-car experiment in 2012, encouraging automakers to develop models to run in a few cities while collecting data on their economic and environmental impacts over the next six years. The conclusion was that methanol cars can be 21% more energy efficient than gas cars while emitting 26% less carbon dioxide. After that pilot phase, the Chinese national government released a policy in 2019 that affirmed its support for methanol fuel, particularly in public transport, taxis, and government vehicles.
In February 2023, an agreement between SFC Energy (SFC)- German and FCTecNrgy Pvt Ltd (FCT)- India was signed during the meeting between German Chancellor Olaf Scholz and Indian Prime Minister Narendra Modi, signifying the commencement of fuel cell production in India.
Significance of Electrocatalysts in Fuel Cell Performance
Initially, platinum (Pt) was commonly used as the catalyst for both half-reactions. However, despite their low efficiency, DMFCs have found niche applications, particularly in portable devices where energy and power density take priority over efficiency concerns. One of the primary drawbacks of DMFCs is the low efficiency associated with the use of platinum catalysts. This limitation restricts their application to situations where compact and lightweight power sources are essential.
The disadvantages include Methanol cross-over (through the membrane leads to the loss of fuel to the cathode side), highly precious metal catalysts, catalyst poisoning, sluggish electrochemical reactions, CO₂ removal, etc. So, the construction of DMFCs needs improvements by utilizing appropriate catalysts for better advancements. The efficiency and prolonged cycle life are determined through its archetypical electrode materials. Inferring the nature of the active sites and incorporation of innovative constituents over pioneering synthetic approaches would contribute to significant developments in such catalysts. Hence, intensive consideration must be given to identify the extremely active electrode materials to enhance their ability.
Importance of Nanoarchitected Catalysts in Advanced Energy Systems
The breakthrough of synthetic methodologies and structural design for advanced materials provides incredible occasions to tune their functionalities in energy conversion and storage systems. The role of dimensionality is also an essential factor for the material’s property due to the easy movements of electrons/ions through its unique structures. As a sign of the large surface area, the usage of nanomaterials as an electrode will improve surface reactivity, and hence the efficiency of energy production is also enhanced.
Nanoarchitected Materials in Electrochemistry
Nanoarchitected materials integrated electrodes minimize the loss of interfacial resistance and improve the kinetics of the reaction. So, developing new nanoarchitectures through optimizing the morphology, structure, size of building blocks of nanomaterials, and the parameters for material processing are always anticipated to respond to the influence of boundaries on the transport of electrons. The encircled high-energy surfaces and well-defined atomic arrangement might offer a promising platform to explore the surface structure-catalytic functionality at nanoscales. For this, the methods that could precisely and endlessly control the surface structure of material formation. The functional units can dramatically change the original surface energy by binding with surface atoms. Furthermore, the low coordinated sites can be improved in stabilization through the capping agents that are slowing the growth rate to the high-energy surface direction and conserving the high-energy catalytic surface.
Concluding Insights and Future Outlook
In summary, the evolution of fuel cell technology, particularly DMFCs, is driven by the need for clean and efficient energy solutions. The adoption of methanol as a fuel, coupled with advancements in catalysts, especially nanoarchitected materials, holds the potential to address efficiency and environmental concerns, contributing to a more sustainable energy future. Despite achieving remarkable electrocatalytic performances, there is a persistent need to enhance the efficacy and viability of synthetic approaches, emphasizing non-noble metal-based electrocatalysts with diverse structures for broader applications. The integration of materials and mechanisms, coupled with cost-effective synthesis, holds the key to achieving superior performance in DMFCs, prompting a focus on understanding how material functions can be optimized through nanoarchitectural design. As technology advances and research progresses, the role of fuel cells is expected to expand further, contributing to a more sustainable and resilient energy future.
Source:
- https://www.technologyreview.com/2022/09/30/1060508/china-betting-methanol-cars/
- https://etn.news/buzz/sfc-energy-hydrogen-methanol-fuel-cell-manufacturing-india-facility
- https://dst.gov.in/cobalt-platinum-alloy-spiced-manganese-effective-catalyst-methanol-oxidation-reaction-methanol-fuel
- https://pubs.acs.org/doi/10.1021/acsami.3c01140
- https://www.bitsathy.ac.in/direct-methanol-fuel-cell/