Dynamic Hydrogen Production from Methanol using Ruthenium Catalyst
The global energy needs are increasing day by day to cater to the needs of the growing population. In spite of being the major energy source, conventional fossil fuels have a strong negative impact on the environment. To mitigate this, biomethanol can be produced from waste biomass like agro wastes, forest litter, and urban organic wastes, after a judicious pre-treatment of complex lignocellulose biomass as a source substrate by various biotechnological and chemical routes. Generally, methanol is produced via syngas in the thermochemical process using lignocellulose by gasification Process. Hydrogen is considered one of the clean energy sources to produce value-added chemicals, Pharmaceuticals, and fuel cell applications. In the future Hydrogen gas is going to be a major fuel and hope for the energy transition in the transport sector. So far Hydrogen can be produced from different sources in different ways to use as a fuel for transportation by common methods such as steam-methane reforming and electrolysis, which are highly expensive and same time difficult to store hydrogen for energy-oriented applications. But at present, due to some limitations in storing hydrogen gas in advanced materials or even transporting gas itself is a complex process. To overcome this difficulty, the process of deriving molecular hydrogen from derived alcohols is known as a methanol-reformer. But conventional reformers still come with a number of drawbacks of catalyst attrition too.
Methanol Reforming
Storing methanol is easier than hydrogen since it does not require high pressures or low temperatures. Since it is a liquid, methanol is easier to transport and use. The production of hydrogen from methanol spontaneously involves a chemical reaction known as methanol-reforming. This reaction requires a new catalyst to facilitate the conversion of methanol into hydrogen and carbon dioxide. This paves the ground for the production of green hydrogen in sunny regions and its conversion into methanol and its simplified transport.
Different types of catalyst and their drawbacks
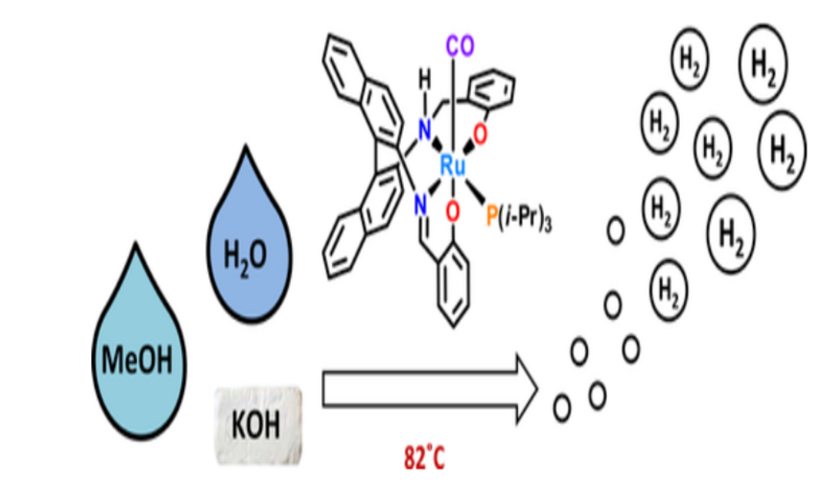
For this conversion of methanol into hydrogen gas several inorganic catalysts have been used from past to present, for example, they are comprised of copper, aluminum, chromium, nickel, platinum-based catalyst, or a combination form, the catalyst powder is added to the reactor in the form of extruded pellets for methanol reforming process. However, these catalyst leads to catalyst attrition that contaminates the fuel cell or entire cell. Further, this catalyst material is also not fully utilized and even the reaction rate, selectivity, and sensitivity are very low. The cost and preparation of the above-mentioned catalyst are highly expensive. Another Challenging problem involved in the mentioned catalytic reactor is heat management, the reactor requires a heat supply to drive the steam reforming reaction, for this is where a lot of efficiency is lost. Hence in order to improve the efficiency a new type of ruthenium-based metal complexes was invented by the Indian Institute of Science Education and Research, at Tirupathi.
Source:https://pubs.aip.org/aip/adv/article/13/3/030701/2879542/A-review-study-on-methanol-steam-reforming
Uniqueness of Ruthenium catalyst
This new technology involves synthesis, characterization of innovative hierarchical Catalysts of ruthenium compounds, and application of producing Hydrogen gas in an eco-friendly, cost-effective one that can operate at ambient temperature. The reason for selecting Ruthenium metal is because Ruthenium serves as a unique catalyst in reactions due to its variable oxidation state which ranges from +2 to +8. Ru exists in two stable oxidation states (II and III), that can be linked with auxiliary ligands of various geometries complexes with varied electronic and steric properties. Methanol reformers convert easy-to-transport methanol into hydrogen. (Source: https://www.sciencedirect.com/science/article/pii/S1878535222004816)
Mechanism of catalytic reaction
The choice of catalyst depends on various factors, including the specific reaction conditions, desired reaction rate, catalyst stability, and cost considerations. The basic chemical reaction for methanol steam reforming is as follows:
CH3OH + H2O → 3H2 + CO2
In this reaction, methanol reacts with water in the presence of a catalyst to produce hydrogen and carbon dioxide as called a dehydrogenation reaction. Ruthenium catalyst helps lower the activation energy for this reaction, making it more feasible under the required operating conditions. This catalyst can facilitate dehydrogenation reactions of various hydrocarbons, such as propane or ethanol to generate hydrogen. It possesses higher selectivity, higher product formation, thermal stability, resistance to catalytic poisoning, good versatility, and environmentally friendly processes.
The molecular structure of a ruthenium catalyst for a dehydrogenation reaction can vary depending on the specific catalyst and the reaction conditions. Usually, ruthenium catalysts are supported on a solid material metal oxide like alumina or silica, and the ruthenium atoms are typically dispersed as nanoparticles or as single atoms on the supported surface. The pattern of dispersion of ruthenium atoms on the support can produce effective reactive sites for reactants and determines the catalyst’s activity, selectivity, and stability in dehydrogenation reactions. Researchers often tailor the catalyst structure to optimize its performance. The sequence involved in catalytic reaction is as follows in five steps such as 1. Adsorption of Methanol2.Activation typically involves the breaking of a hydrogen-carbon (H-C) bond.3. Dehydrogenation: The ruthenium-methoxide intermediate undergoes dehydrogenation where the hydrogen atom is removed. 4. Desorption of Formaldehyde5. Hydrogen evolution. The hydrogen atom (H*) that was formed during the dehydrogenation step evolved as hydrogen gas (H2)
Factors influencing the catalytic reactions
The rate of conversion can be increased by the following factors creating Nano nano-sized catalyst having a higher surface area, optimum reaction temperature, pressure, amount of catalyst loading, presence of promoters, higher concentration and purity of methanol, and proper heat transfer mechanism. Overall this value-added product is socially beneficial to the farmers through agro-based industries and reduces global warming, acid rainfall, or ozone layer depletion as one of the green initiatives for achieving sustainable development goals.
Source: